Скачать с ютуб London Dispersion Forces, Dipole Interactions, and Hydrogen Bonds в хорошем качестве
Из-за периодической блокировки нашего сайта РКН сервисами, просим воспользоваться резервным адресом:
Загрузить через ClipSave.ruСкачать бесплатно London Dispersion Forces, Dipole Interactions, and Hydrogen Bonds в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно London Dispersion Forces, Dipole Interactions, and Hydrogen Bonds или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон London Dispersion Forces, Dipole Interactions, and Hydrogen Bonds в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
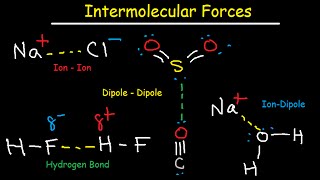
London Dispersion Forces, Dipole Interactions, and Hydrogen Bonds
Need help preparing for the General Chemistry section of the MCAT? MedSchoolCoach expert, Ken Tao, will teach everything you need to know about London dispersion forces, dipole interactions, and hydrogen bonds of intermolecular forces. Watch this video to get all the mcat study tips you need to do well on this section of the exam! Intermolecular forces are the everyday interactions mediating the attraction between all molecules. The forces are termed electrostatic because they are the result of the attraction between a positive charge and a negative charge. The attractive force between two oppositely charged particles can be understood through Coulomb’s Law, from which we know that when a molecule contains a greater magnitude of charge it has the capacity to experience a greater electric force. The MCAT exam expects an understanding of three primary intermolecular forces: London dispersion forces, dipole-dipole forces, and hydrogen bonding. London Dispersion Forces Generally considered the weakest of the three forces, London Dispersion Forces (LDFs) are a universal component of the interactions between every pair of molecules. LDFs occur when the electron cloud of a molecule temporarily undergoes a disturbance, resulting in the formation of partial positive and partial negative charges throughout a molecule which allow it to interact with neighboring dipoles. The charge of a molecule can be understood by thinking about its electron cloud, which is a term describing the possible location of every electron contained within a molecule. If the electrons around the molecule are distributed unevenly (i.e. one part of the molecule has many more electrons than another), then the molecule can be said to have a dipole. The existence of a dipole implies that some parts of the molecule are more positive or more negative than others. Some molecules contain a dipole under resting conditions, and these molecules are termed polar. On the other hand, nonpolar molecules contain a dipole only when their electron clouds are disturbed by some external force. When any molecule – polar or nonpolar – undergoes a disturbance to its electron cloud, the resultant temporary dipole allows the molecule to interact with neighboring dipoles. The positive end of one atom’s temporary dipole may interact with the negative end of a nearby atom’s temporary dipole. Because temporary dipoles are created through the effect of an external electric charge on a particular electron cloud, these dipoles are termed “instantaneous dipole-induced dipole forces”. These temporary dipoles will eventually disappear as the molecule returns to normal. LDFs are typically considered the weakest of the IMFs, but larger molecules generally produce greater LDFs than smaller molecules. The ability for a molecule’s electron field to become disturbed by an external force is its polarizability. Recall that traveling down a group within the periodic table, atoms with a greater number of electron shells have a weaker Zeff, because the electrons farthest out experience a reduced attractive force from that atom’s nucleus. Therefore, within a group, the most distant electrons of large atoms such as Iodine will be more easily disturbed than the most distant electrons of small atoms such as Fluorine. This principle generally holds true for large molecules: the bigger the molecule, the more polarizable it’s electron cloud, the greater the dipoles it will form, and the stronger the LDFs it will produce with neighboring dipoles. Dipole-Dipole Forces We noted that some molecules contain a dipole under resting conditions, and these molecules are termed polar. These molecules contain a permanent separation of partial positive and negative charges, which would not disappear in the absence of external electric forces. The spatial distribution of these charges is heterogeneous around the atom, meaning that certain parts of the atom will be permanently somewhat positive, or permanently somewhat negative. Given these permanent dipoles, a polar molecule will always be able to interact with any neighboring dipoles. For example, HCl, a polar molecule, will always be able to interact with any neighboring polar molecules via dipole-dipole forces. MEDSCHOOLCOACH To watch more MCAT video tutorials like this and have access to study scheduling, progress tracking, flashcard and question bank, download MCAT Prep by MedSchoolCoach IOS Link: https://play.google.com/store/apps/de... Apple Link: https://apps.apple.com/us/app/mcat-pr... #medschoolcoach #MCATprep #MCATstudytools




![Объяснение 12 законов Вселенной и их Применение в Жизни [ЧТОБЫ ЛЕГКО ПОЛУЧАТЬ ЖЕЛАЕМОЕ]](https://i.ytimg.com/vi/0JH1E6nw1Qc/mqdefault.jpg)



