Скачать с ютуб The Rutherford's Gold Foil в хорошем качестве
Скачать бесплатно The Rutherford's Gold Foil в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно The Rutherford's Gold Foil или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон The Rutherford's Gold Foil в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
The Rutherford's Gold Foil
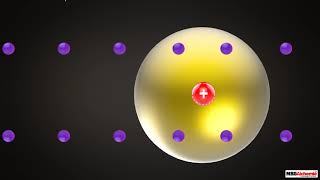
The Rutherford's atomic model posits that atoms have a small, dense nucleus at their center, containing positively charged protons, while electrons orbit around it. This model emerged from Rutherford's gold foil experiment, where he observed that most alpha particles passed through the foil, suggesting a mostly empty space within atoms. Click the join or thanks button to get involved in making science more interesting and available to every human being! Support us on Patreon: / freemededucation Help us making Education Universal: https://www.Launchgood.com/educationf... We believe that learning should be accessible, engaging, and inspiring for all. Our aim is to empower learners of all ages and backgrounds to explore the wonders of science, and to ignite a lifelong passion for learning. With our state-of-the-art resources and captivating animations, we're bringing education to life! Join us and let's create a brighter, more connected world through the power of learning. Follow our Social Media: Facebook: / freeanimatededucation CREDITS: Director: Ramadhan Istabaq Supervisor: Tubagus MP Researcher: Risa Rafika Sari Storyboard Artist: Dwi Yunanto Illustrator: Hussein Shibghotulloh Animator: Fajar Yudoyono, Tubagus MP SFx Designer: Rafly Moravia Subtitle: Risa Rafika Sari Narrator: Nyzzig #FreeAnimatedEducation #rutherfordatomicmodel #rutherfordmodel #rutherfordscatteringexperiment #rutherford #atom #atomicmodel #nucleus #cesium #goldfoil Timestamp (EN): 00:00 Introduction 00:44 Early atomic model: Plum Pudding 01:10 The shortcomings of Plum Pudding Model 01:26 Rutherford's experiment setup 02:08 The result from Rutherford's experiment 02:34 Rutherford's idea and his atomic model 03:11 The shortcomings of Nuclear Model