Скачать с ютуб Metabolite Extraction from Bacterial Culture в хорошем качестве
Скачать бесплатно Metabolite Extraction from Bacterial Culture в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно Metabolite Extraction from Bacterial Culture или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон Metabolite Extraction from Bacterial Culture в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
Metabolite Extraction from Bacterial Culture
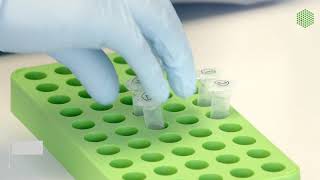
Demonstration of our protocol for collecting metabolites from bacterial cultures. For more info on accurate sample collection, try • Getting a representative "snapshot" o... Transcript: Welcome to the DAN lab! Today we are demonstrating our protocol for extracting intracellular metabolites from bacterial cultures. This protocol requires some preparation before you dive in. To make the extraction solvent, combine HPLC-grade acetonitrile, methanol, and water at a 2:2:1 ratio. Chill the solvent to -20C. Use clean forceps to separate the filters you’re going to use during the extraction. This lets you move through the extraction protocol much more quickly. Discard the blue papers that separate each filter and place the filters in a petri dish. Be sure to prepare a few extras. Label a small petri dish for each extraction you will do. Place these on dry ice or a -80C aluminum block to chill. Take out your chilled extraction solvent and aliquot 1.5mL of extraction solvent into each chilled petri dish. Keep these on dry ice or, again, a -80C aluminum block. The extraction solvent is highly volatile, so you want to keep it cold at all times. Next, set up for your extractions. You’ll need: - 5ml pipet and tips OR a pipettor and serological pipets - Your separated filters and clean forceps - A sintered glass funnel with a rubber stopper and compatible vacuum flask - A vacuum pump - A squirt bottle full of deionized water - Cooler full of dry ice or -80C aluminum block holding your chilled solvent in small petri dishes. Do not use dry ice if you are doing extractions in an anaerobic chamber because it releases CO2 in the chamber, which you don’t want. - Not pictured: o A glass bottle of HPLC-grade water o Your -20C extraction solvent o Bacterial cultures to extract from (but don’t grab those just yet) Attach the vacuum flask to the vacuum pump with tubing. Insert the glass funnel. Make sure everything is secure here, then turn on the vacuum pump. Rinse the funnel with your cold extraction solvent, then rinse it again with water to make sure it’s nice and clean. Then you’re ready to extract! Rinse your forceps and grab one filter disk. Place the filter disk on the glass funnel so that it covers the porous surface completely. Use your forceps to hold and adjust the filter as needed. Rinse the filter with deionized water and make sure that it is laying flat on the glass filter. This could also be done with a serological pipet, or the squirt bottle full of water if there are no concerns about plastic contamination. Make sure that the filter is damp before you add your culture. Grab the petri dish for your first extraction sample out of your cooler, and then grab your culture. Pipet 5mL of bacterial culture onto the center of the filter disk. Be careful that none of the culture goes over the side of the funnel or past the edge of the filter disk, ensuring that you collect metabolites from exactly 5mL of culture. Remove the filter from the funnel and place it cell-side DOWN into your dish of solvent. Swirl the dish to evenly distribute solvent across the whole thing. Return the dish to your cooler with either dry ice or your -80C aluminum blocks. Rinse the funnel and your forceps with water between each sample. Repeat the extraction with a new filter disk for every culture, returning the dishes of solvent back to your cooler when not in use. Make every effort to minimize the amount of time that the culture spends outside of its growth condition because cellular metabolism changes rapidly in response to changing environmental conditions! Repeat the extraction protocol for every culture before moving on to the next steps. Store your petri dishes full of solvent with the filters in your cooler until you’re done. When you’ve finished, give the glass filter a final rinse with water and then turn off the vacuum pump. After you’ve finished all the extractions, you need to transfer your solvent with extracted metabolites into eppy tubes. Take each dish out of the cooler one at a time. Rinse the filter disk thoroughly by pipetting the solvent over the disk, about five times. Flip the disk over with forceps and repeat this rinsing on the other side. When you are finished rinsing, transfer the liquid into a clean, labeled Eppendorf tube. Repeat this for all of your samples. Spin the samples down in a microcentrifuge to remove cellular debris. Spin for five minutes at maximum speed. Remove the tubes back to ice when your spin is finished. Without disrupting the pellet, transfer the desired volume of clear supernatant to a clean, labeled tube. Discard the pellet. These samples should be stored at -80C if not used immediately. Music: bensound.com








![Объяснение 12 законов Вселенной и их Применение в Жизни [ЧТОБЫ ЛЕГКО ПОЛУЧАТЬ ЖЕЛАЕМОЕ]](https://i.ytimg.com/vi/0JH1E6nw1Qc/mqdefault.jpg)