Скачать с ютуб lactate dehydrogenase в хорошем качестве
Скачать бесплатно lactate dehydrogenase в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно lactate dehydrogenase или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон lactate dehydrogenase в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
lactate dehydrogenase
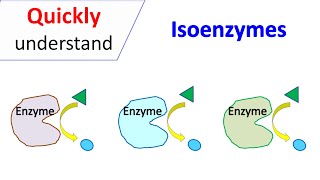
(LDH, LD) An enzyme found in nearly all living cells. It catalyzes the conversion of lactate to pyruvate and back, as it converts NAD+ to NADH and back. A dehydrogenase is an enzyme that transfers a hydride from one molecule to another. All tissues contain various amounts of the 5 LDH isoenzymes. However, muscle, liver, and red blood cells are the major sources of serum LDH activity. LDH isoenzymes (LDH-1, LDH-2, LDH-3, LDH-4, and LDH-5) are made up of different ratios of LDH-M (muscle type) and LDH-H (heart type) subunits, transcribed from LDHA and LDHB, respectively. The LDHC tetramer is only made up of LDHC subunits. LDH isoenzymes: - LDH-1: Present primarily in cardiac myocytes and erythrocytes. - LDH-2: Present mostly in white blood cells. - LDH-3: Present in highest quantity in lung tissue. - LDH-4: Highest amounts found in pancreas, kidney, and placenta. - LDH-5: Highest amounts found in liver and skeletal muscle. They are normally localized in the cytosol and released by damaged cells. Thus, the concentration of LDH in the cell supernatant is an indicator for cell damage. They were used widely in the past for diagnosis of myocardial infarction, but more recently, due to availability of troponin immunoassays, lactate dehydrogenase isoenzyme assay has been mostly discontinued in the clinical setting for diagnosis of myocardial infarction. However, it may be used in evaluating certain hepatic disorders.