Скачать с ютуб 9.5 Molecular Orbital Theory | General Chemistry в хорошем качестве
Из-за периодической блокировки нашего сайта РКН сервисами, просим воспользоваться резервным адресом:
Загрузить через ClipSave.ruСкачать бесплатно 9.5 Molecular Orbital Theory | General Chemistry в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно 9.5 Molecular Orbital Theory | General Chemistry или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон 9.5 Molecular Orbital Theory | General Chemistry в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
9.5 Molecular Orbital Theory | General Chemistry
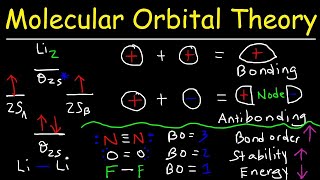
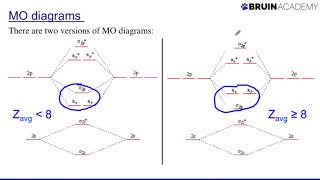
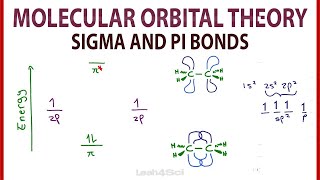
Chad provides a comprehensive lesson on Molecular Orbital Theory. The lesson begins by showing how overlap of atomic orbitals results in both bonding and antibonding molecular orbitals. Constructive overlap between atomic orbitals results in a lower energy, bonding molecular orbital, while destructive overlap between atomic orbitals results in a higher energy, antibonding molecular orbital. The Molecular Orbital diagrams of H2 and He2 are both presented and used to explain why H2 exists and He2 does not. It is also shown how to calculate bond order from a molecular orbital diagram (formula) and how for many species this is consistent with the number of bonds in the Lewis structure. The Molecular Orbital diagrams for homonuclear diatomic molecules of the second period are then presented. The Molecular Orbital diagrams of O2, F2, and Ne2 are presented and the corresponding bond orders calculated. Paramagnetic and diamagnetic are defined and it is shown that a species is paramagnetic if it has unpaired electrons and diamagnetic if all of its electrons are paired. The MO diagrams are then used to explain why Ne2 does not exist (bond order = 0) and why O2 is paramagnetic, something that wouldn't be predicted by the Lewis structure. The MO diagram for N2 is then presented and it is shown that the energies of the sigma 2p and pi 2p molecular orbitals are inverted due to sp mixing in the MO diagram for Li2, Be2, B2, C2, and N2. The bond order for N2 is calculated (it equals 3) which corresponds nicely with the triple bond present in the Lewis structure. I've embedded this playlist as a course on my website with all the lessons organized by chapter in a collapsible menu and much of the content from the study guide included on the page. Check this lesson out at https://www.chadsprep.com/chads-gener... If you want all my study guides, quizzes, final exam reviews, and practice exams, check out my General Chemistry Master Course (free trial available) at https://www.chadsprep.com/genchem-you... 00:00 Lesson Introduction 01:09 Constructive & Destructive Overlap 05:26 Sigma 1s & 1s* 14:30 Sigma 2p & 2p* 19:58 Pi 2p & 2p* 24:34 Molecular Orbital Diagram for H2 26:29 Molecular Orbital Diagram for He2 27:17 How to Calculate Bond Order from Molecular Orbital Diagram 30:58 Molecular Orbital Diagram for O2, F2, Ne2 35:22 Paramagnetic vs Diamagnetic 39:54 Molecular Orbital Diagram for N2 https://www.chadsprep.com/ https://courses.chadsprep.com/pages/p...