Скачать с ютуб Hypoxia Inducible Factors (HIFs), Part 2: Regulation of HIF under normoxic and hypoxic conditions в хорошем качестве
Из-за периодической блокировки нашего сайта РКН сервисами, просим воспользоваться резервным адресом:
Загрузить через ClipSave.ruСкачать бесплатно Hypoxia Inducible Factors (HIFs), Part 2: Regulation of HIF under normoxic and hypoxic conditions в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно Hypoxia Inducible Factors (HIFs), Part 2: Regulation of HIF under normoxic and hypoxic conditions или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон Hypoxia Inducible Factors (HIFs), Part 2: Regulation of HIF under normoxic and hypoxic conditions в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
Hypoxia Inducible Factors (HIFs), Part 2: Regulation of HIF under normoxic and hypoxic conditions
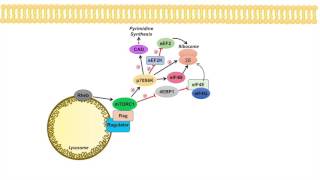
The Hypoxia Inducible Factor (HIF) is a transcription factor, which consists of an alpha and a beta subunit. One of three alpha subunits dimerizes with one of two beta subunits. The alpha subunits are sensitive to oxygen, whereas the beta subunits are not. In this video, the domain structure of an HIF1α subunit is shown. A domain in the center of the protein contains two critical proline residues, which are important for the stability of the protein. An arginine residue in the C-terminal transactivation domain regulates transcriptional activity. Under normoxic conditions, the tissue concentration of molecular oxygen is in the range of 10 to 30 µM. Under these conditions, a member of the prolyl hydroxylase domain family (PHD) hydroxylates one or both of the critical proline residues in the HIFα proteins. When a single or both of the critical proline residues have been hydroxylated, the von Hippel Lindau protein (pVHL), a tumor suppressor protein, binds to HIFα. The von Hippel Lindau protein associates with a ubiquitin ligase complex that causes the attachment of ubiquitin to HIFα. Polyubiquitinated HIFα is degraded by the 26S proteasome. In addition to the proline residues, HIFα proteins contain a critical arginine residue in the C-terminal transactivation domain. The residue is hydroxylated by a dioxigenase designated factor inhibiting HIF1α (FIH1). The hydroxylated arginine residue inhibits the association of the p300 and CBP transcriptional co-activators with HIFα. Under hypoxic conditions, the activity of PHD dioxigenases decreases. As a consequence, the proline residues of HIF are no longer hydroxylated, and the HIFα units are stabilized. A HIFα subunit associates with a HIFβ subunit. The heterodimer binds to a consensus sequence in the DNA. Since FIH1 no longer hydroxylates the arginine residue in the C-terminal transactivation domain of HIFα, the p300 and CBP co-activators are able to bind. The heterodimer activates the transcription of target genes. #onkoview, #hypoxia, #hif, #26S, #HIF1α, #genes, #heterodimer, #transcription, #proteins