Скачать с ютуб 7.5 E2 Reactions | Organic Chemistry в хорошем качестве
Скачать бесплатно 7.5 E2 Reactions | Organic Chemistry в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно 7.5 E2 Reactions | Organic Chemistry или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон 7.5 E2 Reactions | Organic Chemistry в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
7.5 E2 Reactions | Organic Chemistry
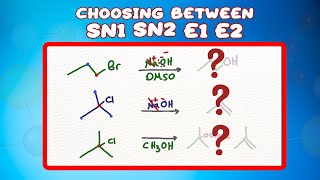
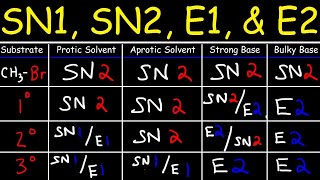
Chad breaks down everything you need to know about E2 Reactions. He starts with the concerted mechanism and the rate law showing the E2 reaction mechanism stereochemistry, how the beta hydrogen and the leaving group must be antiperiplanar when E2 elimination occurs. He introduces Zaitsev Rule (aka Saytzeff Rule), how it is most common in E2 reactions for the most substituted alkene possible to be the major product. He then shows that for some substrates with only one beta hydrogen only one specific stereoisomer may be possible for the product (either E or Z) and shows how to predict which one it is. Chad then explains that for E2 reactions involving cyclohexane the beta hydrogen and the leaving group must be trans-diaxial in order to be antiperiplanar and for the E2 mechanism to occur. He concludes the lesson by showing common exceptions to Zaitsev rule (Hofmann Elimination) such as when a bulky base (like t-butoxide) is used or when having a poor leaving group like fluorine. Want Chad’s Organic Chemistry Study Guides and Practice Quizzes/Tests? Check out Chad’s Ultimate Organic Chemistry Prep. [Free Trial available!] https://www.chadsprep.com/organic-che... 00:00 Lesson Introduction 00:52 E2 Reaction Mechanism and Zaitsev Rule 02:41 E2 Rate Law 03:41 E2 Reaction Stereochemistry - Antiperiplanar 08:43 E2 Reactions Stereochemistry - E vs Z Alkene Product 14:14 E2 Reactions with Cyclohexane 19:10 E2 with a Bulky Base (Hofmann Product) 22:44 E2 with a Bad Leaving Group (Hofmann Product) https://www.chadsprep.com/